Synthesis and Development of Chemiluminescent Soft Crystals for Spatiotemporal Control of the Stimulus-responsive Functions
Co-Investigator: Hiroshi Ikeda (Graduate School of Engineering, College of Engineering, Osaka Prefecture University, Professor)
Co-Researcher: Chiharu Matsuhashi (Graduate School of Informatics and Engineering, The University of Electro-Communications, Researcher)
Research Outline
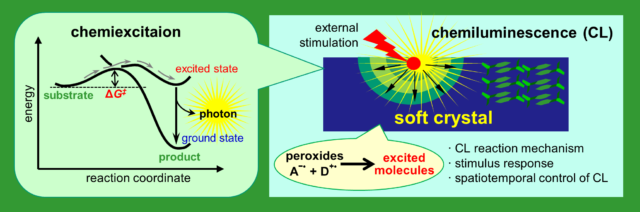
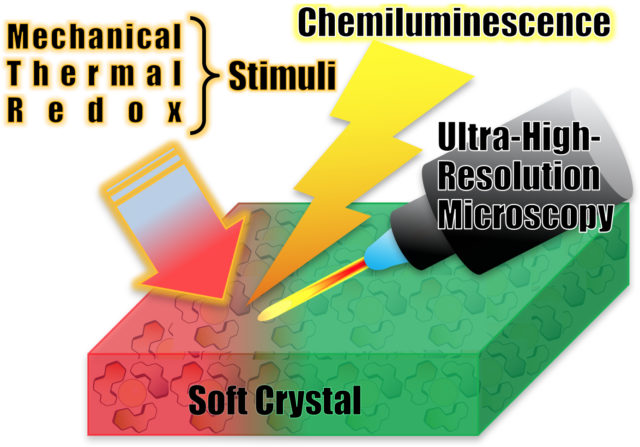
Chemiluminescence (CL) is a phenomenon in which excited molecules are generated by thermochemical reactions to emit light. It would be possible to detect extremely minute structural changes in a soft crystal at the atomic and molecular levels as CL changes, since ultrahigh sensitivity analysis is made possible by observing CL as a probe. In this research, we will create soft crystal CL systems that initiate luminescence by external stimulations to crystals. By elucidating the mechanism of stimulus-responsive CL with thermodynamic, kinetic, and quantum theoretical approaches, we also aim for spatiotemporal control of CL produced at arbitrary position and time in soft crystals. Specifically, we will conduct our research in the following two ways, (1) organic peroxides and luminescent redox-active metal complexes will be prepared for CL in soft crystals to establish the requirements for CL-active crystal structures and elucidate the CL reaction mechanisms; (2) We will take advantage of recently-developed ultra-high-resolution microscopy and time-resolved spectroscopy based on full-time “in-situ” observations of stimulus CL responses.

Representative Achievements
- Spectroscopic Properties of Amine-substituted Analogues of Firefly Luciferin and Oxyluciferin
Kakiuchi, M.; Ito, S.; Yamaji, M.; Viviani, V. R.; Maki, S.; Hirano, T. Photochem. Photobiol. 2017, 93, 486–494.
DOI: 10.1111/php.12654 - Spectroscopic Properties of BF2 Complexes of N-(5-Phenyl-2-pyrazinyl)pivalamides Exhibiting Fluorescence in Solution and Solid State
Hachiya, S.; Hashizume, D.; Ikeda, H.; Yamaji, M.; Maki, S.; Niwa, H.; Hirano, T. J. Photochem. Photobiol. A 2016, 331, 206–214.
DOI: 10.1016/j.jphotochem.2015.10.014 - Catalytic Formation of Hydrogen Peroxide from Coenzyme NADH and Dioxygen with a Water-Soluble Iridium Complex and a Ubiquinone Coenzyme Analogue
Suenobu, T.; Shibata, S.; Fukuzumi, S. Inorg. Chem. 2016, 55, 7747–7754.
DOI: 10.1021/acs.inorgchem.6b01220 - Catalytic hydrogen Production from Paraformaldehyde and Water Using an Organoiridium Complex
Suenobu, T.; Isaka, Y.; Shibata, S.; Fukuzumi, S. Chem. Commun. 2015, 51, 1670–1672.
DOI: 10.1039/C4CC06581F - Chemiluminescent 2,6-Diphenylimidazo[1,2-a]pyrazin-3(7H)-ones: a New Entry to Cypridina Luciferin Analogues
Ishii, Y.; Hayashi, C.; Suzuki, Y.; Hirano, T. Photochem. Photobiol. Sci. 2014, 13, 182–189.
DOI: 10.1039/C3PP50197C
Publications
Academic papers/reviewed
- "Intracrystalline kinetics analyzed by real-time monitoring of a 1,2-dioxetane chemiluminescence reaction in a single crystal" C. Matsuhashi, H. Fujisawa, M. Ryu, T. Tsujii, J. Morikawa, H. Oyama, H. Uekusa, S. Maki, *T. Hirano, Bull. Chem. Soc. Jpn. 95, 413-420 (2022). (領域内共同研究 A01 公募 植草, A03 公募 森川)
- "Aggregation-induced emission active thermally-activated delayed fluorescence materials possessing N-heterocycle and sulfonyl groups" *Y. Matsui, Y. Yokoyama, T. Ogaki, K. Ishiharaguchi, A. Niwa, E. Ohta, M. Saigo, K. Miyata, *K. Onda, *H. Naito, *H. Ikeda, J. Mater. Chem. C, in press. (doi.org/10.1039/D1TC05196B). (Cover) (領域内共同研究 A03-01 恩田・宮田・石井) イシイDOI: 10.1039/D1TC05196B
- "A stable chemiluminophore, adamantylideneadamantane 1,2-dioxetane: from fundamental properties to utilities in mechanochemistry and soft crystal science" *T. Hirano, C. Matsuhashi, J. Photochem. Photobiol. C, 51, 100483 (13 pages) (2022).
- "Self-assembly of non-macrocyclic triangular Ni3Ln clusters" T. N. Dais, R. Takano, T. Ishida, *P. G. Plieger, Dalton Trans., 51, 1446-1453 (2022).DOI: 10.1039/D1DT03742K
- "Metallocyclic CuII-LnIII single-molecule magnets from the self-assembly of 1,4-diformylnaphthalene-2,3-diol" T. N. Dais, R. Takano, Y. Yamaguchi, T. Ishida, *P. G. Plieger, ACS Omega, 7, 5537-5546 (2022).
- "Lanthanide induced variability in localised CoII geometries of four triangular L3Co3IILnIII complexes" T. N. Dais, R. Takano, T. Ishida, *P. G. Plieger, RSC Adv., 12, 4828-4835 (2022).DOI: 10.1039/D1RA08797E
- "Singlet-oxygen chemiluminescence from heated crystal samples of 9,10-diphenylanthracene endoperoxides" N. Yamasaki, C. Matsuhashi, S. Maki, *T. Hirano, Chem. Lett., 50, 1681-1683 (2021).
- "Development of phenyl oligoene-type firefly luciferin analogues with extended π-electronic conjugation for near-infrared bioluminescence" G. Kamiya, N. Kitada, R. Saito-Moriya, R. Obata, S. Iwano, A. Miyawaki, T. Hirano, *S. Maki, Chem. Lett., 50, 1523-1525 (2021).DOI: 10.1246/cl.210261
- "Synthesis of novel π-extended D–A–D-type dipyrido[3,2-a:2’,3’-c]phenazine derivatives and their photosensitized singlet oxygen generation" Y. Hayashi, A. Morimoto, T. Maeda, T. Enoki, Y. Ooyama, Y. Matsui, H. Ikeda, *Y. Yagi, New J. Chem., 45, 2264-2275 (2021).DOI: 10.1039/D0NJ05526C
- "Elongation of triplet lifetime caused by intramolecular energy hopping in diphenylanthracene dyads oriented to undergo efficient triplet–triplet annihilation upconversion" M. Kanoh, *Y. Matsui, K. Honda, Y. Kokita, T. Ogaki, E. Ohta, *H. Ikeda, J. Phys. Chem. B, 125, 4831-4837 (2021). (Cover)
- "Polymeric terbium (III) squarate hydrate as a luminescent magnet" R. Takano, *T. Ishida, Crystals, 11, 1221 (9 pages) (2021).
- "Color-Tunable Bioluminescence Imaging Platform for Cell Imaging" S. Tamaki, N. Kitada, M. Kiyama, R. Fujii, T. Hirano, *S. B. Kim, *S. Maki, Sci. Rep., 11, 2219 (10 pages) (2021).
- "Synthesis of polyenylpyrrole derivatives with selective growth inhibitory activity against T-cell acute lymphoblastic leukemia cells" C. Yoshida, T. Higashi, Y. Hachiro, Y. Fujita, T. Yagi, A. Takechi, C. Nakata, K. Miyashita, N. Kitada, R. Saito, R. Obata, T. Hirano, T. Hara, *S. A. Maki, Bioorg. Med. Chem. Lett., 37, 127837 (4 pages) (2021).
- "A very bright far-red bioluminescence emitting combination based on engineered railroadworm luciferase and 6'-amino-analogs for bioimaging purposes" *V. R. Viviani, V. R. Bevilaqua, D. R. Souza, G. F. Pelentir, M. Kakiuchi, T. Hirano, Int. J. Mol. Sci., 22, 303 (13 pages) (2021).DOI: 10.3390/ijms22010303
- "Molecular S = 2 High-Spin, S = 0 Low-Spin and S = 0 ⇄ 2 Spin-Transition/-Crossover Nickel(II)-Bis(nitroxide) Coordination Compounds" *T. Ishida, S. Ito, Y. Homma, Y. Kyoden, Inorganics, 9, 10 (28 pages) (2021). (Cover)
- "Development of near-infrared firefly luciferin analogue reacted with wild type and mutant luciferases" N. Kitada, R. Saito, R. Obata, S. Iwano, K. Karube, A. Miyawaki, T. Hirano, *S. A. Maki, Chirality, 32, 922-931 (2020).DOI: 10.1002/chir.23236
- "Bright near-infrared chemiluminescent dyes: Phthalhydrazides conjugated with fluorescent BODIPYs" G. Li, T. Hirano, *K. Yamada, Dyes Pigm., 178, 108339 (7 pages) (2020).
- "Bridged stilbenes: AIEgens designed via a simple strategy to control the non-radiative decay pathway" R. Iwai, *S. Suzuki, S. Sasaki, A. S. Sairi, K. Igawa, T. Suenobu, K. Morokuma, *G. Konishi, Angew. Chem. Int. Ed., 132, early view (2020).(プレスリリース)
- "Isomeric difference in the crystalline-state chemiluminescence property of an adamantylideneadamantane 1,2-dioxetane with a phthahlimide chromophore" C. Matsuhashi, T. Ueno, H. Uekusa, A. Sato-Tomita, K. Ichiyanagi, S. Maki, *T. Hirano, Chem. Commun., 56, 3369-3372 (2020). (Back Cover) (領域内共同研究 A02-03 佐藤, A01 公募 植草)DOI: 10.1039/C9CC10012A
- "Reaction of Oxygen with the Singlet Excited State of [n]Cycloparaphenylenes (n = 9, 12, and 15): A Time-Resolved Transient Absorption Study Seamlessly Covering Time Ranges from Subnanoseconds to Microseconds by the Randomly-Interleaved-Pulse-Train Method" *T. Suenobu, I. Arahori, K. Nakayama, T. Suzuki, *R. Katoh, *T. Nakagawa, J. Phys. Chem. A, 124, 46-55 (2020). (Cover)
- "Synthesis of fluorescent polycarbonates with highly twisted N,N-bis(dialkylamino)anthracene AIE luminogens in the main chain" A. S. Sairi, K. Kuwahara, S. Sasaki, S. Suzuki, K. Igawa, M. Tokita, S. Ando, K. Morokuma, T. Suenobu, *G.-i. Konishi, RSC Adv., 9, 21733-21740 (2019).DOI: 10.1039/C9RA03701B
- "Halogen-substituent effect on the spectroscopic properties of 2-phenyl-6-dimethylaminobenzothiazoles" R. Misawa, C. Matsuhashi, M. Yamaji, T. Mutai, I. Yoshikawa, H. Houjou, K. Noguchi, S. Maki, *T. Hirano, Tetrahedron Lett., 60, 1702-1705 (2019). (Cover) (領域内共同研究 A01-01分担 務台)
- "Phrixotrix luciferase and 6’-aminoluciferins reveal a larger luciferin phenolate binding site and provide novel far-red combinations for bioimaging purposes" V. R. Bevilaqua, T. Matsuhashi, G. Oliveira, P. S. L. Oliveira, T. Hirano, *V. R. Viviani, Sci. Rep., 9, 8998 (17 pages) (2019).
- "Synthesis and luminescence properties of near-infrared N-heterocyclic luciferin analogues for in vivo optical imaging" R. Saito, T. Kuchimaru, S. Higashi, S. Lu, M. Kiyama, S. Iwano, R. Obata, T. Hirano, *S. Kizaka-Kondoh, *S. A. Maki, Bull. Chem. Soc. Jpn., 92, 608-618 (2019).
- "Spectroscopic properties of push-pull 2-(4-carboxyphenyl)-6-dimethylaminobenzothiazole derivatives in solution and the solid state" Y. Takahashi, T. Uehara, C. Matsuhashi, M. Yamaji, T. Mutai, I. Yoshikawa, H. Houjou, K. Kitagawa, T. Suenobu, S. A. Maki, *T. Hirano, J. Photochem. Photobiol. A., 376, 324-332, (2019). (領域内共同研究 A01-01分担 務台)
- "Structure-fluorescence relationship of push-pull 2-phenylbenzothiazole derivatives designed based on the firefly light-emitter," T. Fujikawa, T. Uehara, M. Yamaji, T. Kanetomo, T. Ishida, S. Maki, *T. Hirano, Tetrahedron Lett., 59, 1431-1434 (2018).
International conferences
- "Study on Soft-Crystal Chemiluminescence, a Solid-State Chemistry to Support Devise Development" T. Hirano (Invited), C. Matsuhashi, F. Koura, S. Maki, H. Uekusa, A. Sato-Tomita, K. Ichiyanagi, M. Ryu, and J. Morikawa, 239th ECS meeting with the 18th International Meeting on Chemical Sensors (IMCS) (Chicago & online, May 30-June 3, 2021).
- "A Soft-Crystal Chemiluminescence System: Luminescence Property of Adamantylideneadamantane 1,2-Dioxetanes Conjugated with a Fluorophore" C. Matsuhashi (Invited), H. Uekusa, K. Ichiyanagi, A. Sato-Tomita, M. Ryu, J. Morikawa, S. Maki, and T. Hirano, 239th ECS meeting with the 18th International Meeting on Chemical Sensors (IMCS) (Chicago & online, May 30-June 3, 2021).
- "Thermal Reaction-induced Phenomena in Soft Crystals Found with 1,2-Dioxetane Chemiluminescence" C. Matsuhashi, H. Oyama, H. Uekusa, H. Fujisawa, M. Ryu, J. Morikawa, A. Sato-Tomita, K. Ichiyanagi, and T. Hirano (Invited), 3rd International Symposium on Soft Crystals jointed with 4th Internal Symposium on Photofunctional Chemistry of Complex Systems and IIS U Tokyo Symposium (Hawaii & online, Dec. 11-15, 2021).
- "Isomeric difference in the crystalline-state chemiluminescence properties of 1,2-dioxetanes with a phthahlimide chromophore," C. Matsuhashi, T. Ueno, H. Uekusa, A. Sato-Tomita, K. Ichiyanagi, J. Morikawa, M. Ryu, S. Maki, T. Hirano, Cooperative phenomena in framework materials: Faraday Discussion (On line, UK, Oct. 13-16, 2020)
- Ishitani, K.; Matsuhashi, C.; Yamaji, M.; Uekusa, H.; Maki, S.; Hirano, T., “Synthesis and chemiluminescence property of 1,2-dioxetanes with an arylmethyl moiety for a soft crystal chemiluminescence system,” The Irago Conference 2018 (Tokyo), P65 (2018.11.1).
- Kitagawa, K.; Suenobu, T.; Nakayama, K., “Selective control of crystal polymorphism of an Mn(II) complex leading to clear difference in emission chromaticity,” The 43rd International Conference on Coordination Chemistry (Sendai), S09-P31 (2018.7.30).
- Suenobu, T; Kitagawa, K.; Nakayama, K., “Vapoluminochromism of Mn(II) complexes in the crystalline state,” The Japan Taiwan Bilateral Workshop on Nanoscience 2018 (Osaka), O−04 (2018.7.21). Invited
- Suenobu, T.; Kitagawa, K.; Nakayama, K.; Katoh, R.; Suzuki, T.; Nakagawa, T., “Aggregation-induced emission of crystalline metal complexes and their vapochromism studied by time-resolved spectroscopies,” The 27th PhotoIUPAC Conference (Dublin), P179 (2018.7.4)
- "The Randomly-Interleaved-Pulse-Train (RIPT) Method for Subnanosecond Transient Absorption Measurement of Metal Complexes" T. Suenobu, T. Suzuki, H. Hanada, H. Hanada, K. Kitagawa, K. Nakayama, T. Nakagawa, R. Katoh, 22nd INTERNATIONAL SYMPOSIUM on PHOTOCHEMISTRY and PHOTOPHYSICS of COORDINATION COMPOUNDS (ISPPCC2017) (Oxford, UK, Jul. 5-7, 2017)
- "The Randomly-Interleaved-Pulse-Train (RIPT) Method for the Measurement of Subnanosecond Transient Absorption Spectra of Photoactive Coordination Compounds" T. Suenobu, The Applications of Photoactive Coordination Compounds conference (APCC2017) (St Andrews, UK, Jul. 10, 2017)