Participating Group (2018-2019)
Study on Nanoporous Molecular Crystals and PCET Conductive Mechanism by Using Hydrogen bonding Metal Complexes
Co-Researcher: You Suzuki (Faculty of Science, Tokyo University of Science, Post Doctor)
Co-Researcher: Kenji Yamane (Faculty of Science, Tokyo University of Science, Post Doctor)
Co-Researcher: Daiki Kan (Faculty of Science, Tokyo University of Science, Post Doctor)
Co-Researcher: Daiki Nagatome (Faculty of Science, Tokyo University of Science, Post Doctor)
Research Outline
In a strong hydrogen bond, the low-barrier hydrogen bond (LBHB) with a low transfer-barrier energy for the proton is often observed in a biological molecule as one of hydrogen-bonds with the same pH values between hydrogen bonding donors and acceptors.
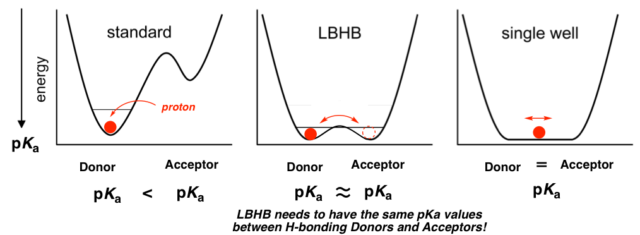
The transition metal complexes connected with LBHB would be easily stabilized mixed-valence states by a H-bonding proton transfer,
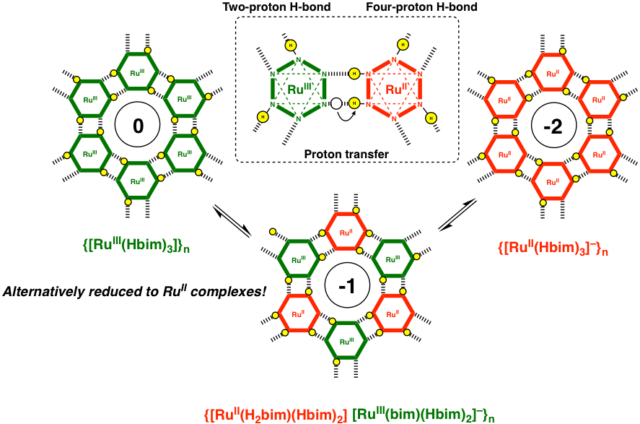
switched by the electrochemical redox reaction.
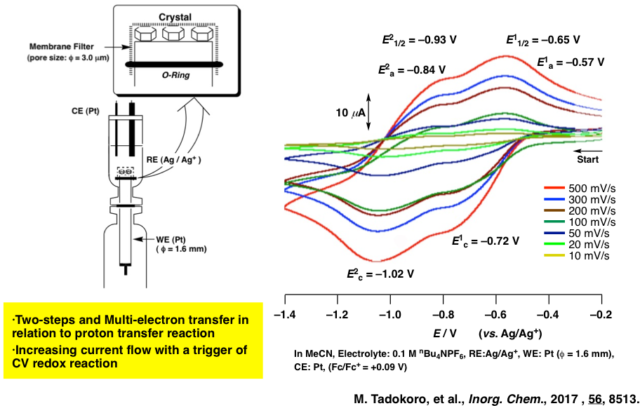
While, each localized state of the PCET (proton-coupled electron transfer) reaction observed through an isolated molecule are affected by solvation in solution. However, in crystal without solvation, the mixed-valance metal complexes connected with LBHB are often observed a PAET (proton-assisted electron transfer) reaction simultaneously transferred both protons and electrons, which has been clarified on a physical property continuously fluctuated a proton and electron. Therefore, we would like to study on evolution of a functional material and elucidation of the mechanism of a PAET crystal. (1) Elucidation of PAET Mechanism by Dimer Complex Crystal with LBHB (2) Study on Molecular Conductor of Honeycomb Sheet Crystal Connected with LBHB (3) Development of PAET Conductor Controlled the Multi-dimensionality. As a result, we would like to elucidate a new molecular material coupled with a proton transfer and electron transfer without solvation.
Publications
Academic papers/reviewed
A03-19
- "Slow Dynamics of Premelting Water Molecules Confined to Hydrophilic Nanoporous Space" Y. Ohata, H. Kamebuchi, K. Watanabe, T. Kouchi, Y. Suzuki, T. Imaizumi, T. Sugaya, M. Mizuno, and M. Tadokoro, Chem. Select, 4, 6627-6633 (2019).
- "Synthesis and electrochromic behavior of a multielectron redox-active N-heteroheptacenequinone" K. Isoda, M. Matsuzaka, T. Sugaya, T. Yasuda and M. Tadokoro, Org. Biomol. Chem., 17, 7884-7890 (2019). プレリリース・カバーピクチャーDOI: 10.1039/C9OB01323G
- "Proton Conduction Inhibited by Xe Hydrates in the Water Nanotube of the Molecular Porous Crystal {{[RuIII(H2bim)3](TMA)}2·mH2O}n" H. Matsui, T. Sasaki, and M. Tadokoro, J. Phys. Chem.C, 123, 20413-20419 (2019).
- "Photo‐Modification of Melanin by a Mid‐infrared Free‐electron Laser" T. Kawasaki, A. Sato, Y. Tominaga, Y. Suzuki, T. Oyama, M. Tadokoro, K. Tsukiyama, K. Nokihara, H. Zen, Photochemistry and Photobiology, 95, 946-950 (2019).DOI: 10.1111/php.13079
- "Dynamic Water Nanotube Cluster Stabilized in Molecule-Based Hydrophilic Nanoporous Crystal with New Organic Spacers" Y. Ohata, K. Takaya, T. Sugaya, H. Kamebuchi, M. Tadokoro, Bull. Chem. Soc. Jap., 2019, 92, 655-660.
- "Evidence of Proton-Coupled Mixed-Valency by Electrochemical Behavior on Transition Metal Complex Dimers Bridged by two Ag+ Ions" M. Tadokoro, K. Isogai, S. Harada, T. Kouchi, T. Yamane, T. Sugaya, H. Kamebuchi, Dalton Trans., 2018, 48, 535-548.DOI: 10.1039/c8dt03962c